What is Breast Implant-Associated Anaplastic Large Cell Lymphoma?
Cosmetic DIEP Flap Procedures with Dr. Sean Boutros
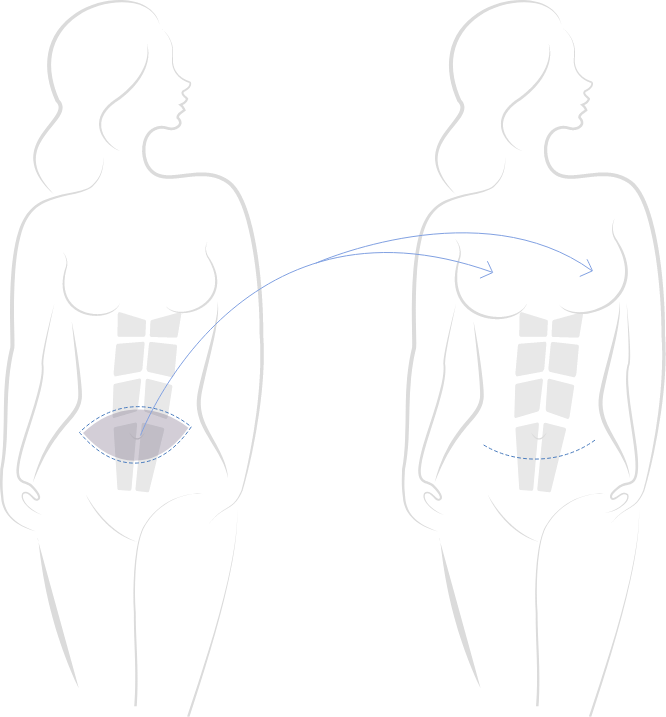
Houston Breast Surgery Specialist Dr. Sean Boutros is a Board Certified Plastic Surgeon and an expert in The All Me Augmentation™ Procedure (Deep Inferior Epigastric Perforator [DIEP] flap surgery). DIEP flap breast surgery has traditionally been a reconstructive procedure for patients who have undergone mastectomy, but Dr. Boutros is revolutionizing cosmetic breast surgery by using DIEP flap methods to provide patients with all natural breast augmentation. Cosmetic DIEP flap breast surgery allows patients to have beautiful, natural-looking breast augmentation without breast implants. This technique uses a patient’s own tissue, harvested from the abdomen, to reshape the breasts. Dr. Boutros offers several breast augmentation options with enhanced recovery and less pain at his Houston practice.
Cosmetic DIEP Flap Breast Augmentation
Many women would like fuller breasts, but do not wish to have breast implants due to safety concerns or personal preference. Cosmetic DIEP flap breast augmentation can deliver results that are more effective than other breast augmentation alternatives, such as fat transfer, without the risks of breast implants. Cosmetic DIEP flap breast augmentation is also an option for patients who would like to have breast implants removed due to complications such as capsular contracture, as this procedure can resolve that condition while allowing patients to maintain full breasts.
Cosmetic DIEP Flap Breast Augmentation with Breast Lift (Mastopexy)
Life changes such as aging, weight fluctuations, and breastfeeding can cause breasts to sag or look deflated. Breast lift surgery combined with breast augmentation can lift and reshape the breasts while also restoring lost volume. Combining cosmetic DIEP flap breast augmentation with breast lift surgery can rejuvenate breasts in a single stage procedure without the potential complications of breast implants.
Mommy Makeover with Cosmetic DIEP Flap Breast Augmentation
For many women, pregnancy and childbirth are the cornerstones of happiness. However, the resulting physical aspects can create long-lasting changes in appearance. A mommy makeover with Cosmetic DIEP Flap Breast Augmentation is a personalized treatment plan from Dr. Boutros that combines cosmetic DIEP flap breast augmentation with tummy tuck surgery and liposuction to restore confidence for women who would like to have a more toned and youthful look.
En Bloc Capsulectomy Breast Implant Removal
Dr. Boutros understands that there are many reasons a woman may decide to have her breast implants removed, from changes in appearance and style to concerns about health. He is sensitive to the needs of each patient and he offers several methods of breast implant removal, including en bloc capsulectomy, which is often the best method for women who require removal due to breast implant complications.